Utilizing Real World Data and Evidence in Clinical Research and Care
Real world data and evidence are playing an increasing role in revolutionizing clinical research studies, allowing more affordable and effective drugs to reach the market faster than ever before.
Real world data (RWD) is genuine patient data that’s derived from a variety of sources like clinical trials, observational studies and patient surveys. RWD can include but is not limited to: electronic health records (EHRs), insurance claims and wearables that indicate a patient’s health status. For example, a patient’s HbA1c levels recorded in an EHR at a primary care clinic is considered RWD. When RWD is analyzed to determine potential benefits or risks of a drug or product, it is called real world evidence (RWE). For example, using HbA1c levels across hundreds of patient EHR records, researchers could hypothetically compare two drugs that have already been approved to find RWE that one drug is significantly more effective at controlling HbA1c levels than another in a subsection of the population.
Below is an overview of how RWD/RWE is currently being utilized to improve outcomes and reduce costs in clinical research and care. We will then review the challenges preventing wider use and adoption of these practices and provide a list of health startups working to alleviate these challenges. Finally, we’ll offer an investment recommendation for the RWD/RWE market.
Utilization in Clinical Research
RWD is helping Sponsors and Contact Research Organizations (CROs) recruit patients for their clinical trials. Clinical trials have traditionally recruited patients through social advertising and clinical trial site surveys. These efforts have mostly been unsuccessful, as shown by a study that found 86% of trials failed to recruit the desired number of patients by their planned deadline. By using RWD, CROs and Sponsors can quickly identify the right patients for a trial by running an analysis of EHR data against their inclusion and exclusion criteria. For example, Flatiron Health has created OncoTrials, a service that allows researchers to quickly search through EHR data to find eligible patients for their trials.
RWD is helping CROs and Sponsors monitor patient safety by providing real-time views of a patient’s health. Historically, there has not been a way to track trial participants when they were away from a trial site. With the rise of digital wearables, trials now can use the RWD they generate to monitor patient safety during the trial. This could help Sponsors anticipate serious adverse events that could potentially delay or halt a trial. Using a device such as the Apple Watch, the Sponsor might notice a significant increase in the resting heart rate of a subgroup of trial participants. By catching this early, Sponsors are enabled to make protocol amendments around inclusion criteria so the trial can continue. According to a 2020 study, over 1,100 clinical trials used a connected product in 2018, with the use of the technology in clinical trials growing at a compound annual growth rate of nearly 34% since the year 2000.
RWD and RWE can also be used to cut trial costs by enabling a hybrid study design. In the past, when a Sponsor conducted a clinical trial, half of the patient population ends up in the control arm of the study, where they do not receive the drug in question. By using RWD/RWE, Sponsors could cut their recruitment needs in half by using data collected from EHR or claims data to replicate the control group. For example, Bristol Myers Squibb (BMS) recently used RWD to construct a synthetic control arm for a regulatory submission to the FDA. Because of this, BMS only had to recruit half of the patients it normally would recruit for a randomized control trial.
Another way these methods can be used is for more cost-effective drug label expansion. Traditionally, in order for an approved drug to expand its prescribed use cases, a Sponsor would have to run a clinical trial to get the efficacy data. Yet through RWD, Sponsors can bypass these high costs and expand the label for an already approved drug. For example, Pfizer’s breast cancer drug Ibrance was approved for women in 2015. Recently, using EHR and claims data showing the effects of the off-label use of Ibrance in men, Pfizer was able to show that the safety profile was similar to the safety profile seen in women without having to run a clinical trial.
Finally, RWE can be used for Pharmaceutical Value-Based Contracting. Currently, the price that manufacturers set is essentially as high as the market will allow. With RWE, pharma may be able to negotiate value-based contracts in which they can take on a certain degree of risk to receive compensation based on the cost savings due to the better outcomes of their invention. For example, UPMC Health plan and AstraZeneca are currently in a value based contract for Brilinta, a drug designed to prevent heart attack and stroke. Payment to AstraZeneca for Brilinta will be tied to the outcomes (lower rate of heart attacks, stroke) Brilinta produces in a subsection of the UPMC Health plan population.
Utilization in Clinical Care
RWD/RWE can help physicians with Clinical Decision Support. Currently, most physicians prescribe pharmaceuticals based on the results of randomized clinical trials. Yet nearly 70% of clinical trial participants in the US are white. This means that a drug’s effect on the real world population may not accurately reflect the trial results. By using RWE, physicians are enabled to prescribe drugs on a more personalized basis. An analysis of EHR data allows a provider to see that one drug may be a better option than another due to how that drug performs in a very specific patient population. For example, Flatiron Assist pulls data from the EHR such as patient age, sex and cancer staging to offer evidence-based therapy options for the provider to discuss with their patients.
This evidence and data allows providers to bridge the gap between clinical care and randomized control trials. Currently, when a patient isn’t responding to the usual treatment options, they may want to explore clinical trials. In the past, providers didn’t have a great way to filter the clinical trials that might work for their patients given the inclusion or exclusion criteria. Utilizing RWD, a patient’s record could be quickly compared against inclusion/exclusion criteria of available trials to enable quick trial enrollment. For example, Flatiron Assist allows providers to match their patients to the appropriate clinical trial based on EHR data.
RWD also enables remote patient monitoring with ease. Historically, there has not been a way to evaluate patients when they were away from a physician’s office. With the rise of wearables, RWD enables providers to ensure there are no life-threatening complications. Using a device such as the Apple Watch, the Sponsor is able to notice a significant decrease in patient activity and check in to avoid possible hospitalization.
Using RWD, providers and payers can run analyses on large amounts of data to discover trends that show a patient could be at risk. Many patients who have high blood pressure and diabetes are at a high risk of developing chronic kidney disease (CKD). Cricket Health is one of the companies taking advantage of this relatively new technology. By analyzing claims data and comparing it to historical data of patients that developed ESRD (kidney failure), payers can determine who is most at-risk and work to prevent the progression of CKD.
Challenges Preventing Further Utilization in Clinical Research and Care
While RWD/RWE could help alleviate the challenges outlined above, the practice is largely hypothetical at this point. In addition to regulatory barriers, there are additional challenges preventing the widespread use of RWD/RWE.
Data collection barriers present a significant hurdle for those wanting to utilize RWD/RWE. Currently, RWD comes from multiple sources: insurance claims data, EHR data, wearables data, pharmacy databases and registries. A single RWD source may not be enough to generate RWE. Therefore, to have enough data on a participant, a CRO/Sponsor would need claims data from different sources. Unfortunately, gaining access to these data points can cost hundreds of thousands of dollars each.
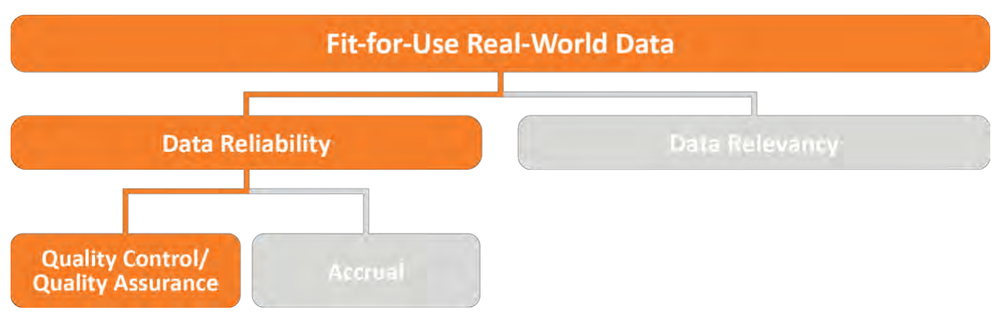
Figure 1: Indicates the steps necessary before data can be considered “Fit-for-Use”. 16
Data analysis barriers present another challenge. Before RWD can be used to create RWE, it must be determined that the data is “fit-for- use,” as shown in Figure 1.
Fit-for-use means the data meets a certain criteria and can be trusted to inform regulatory decision making. In order for data to be determined fit-for-use, the data must have “reliability.” Reliability means the data adequately represent the underlying medical concepts they are intended to represent. To ensure reliability, the RWD must go through the “quality control/quality assurance” phase. Quality control refers to steps that are taken to ensure the data is reproducible. The quality assurance process entails the proactive and retrospective steps taken to evaluate whether pre-specified requirements were fulfilled.
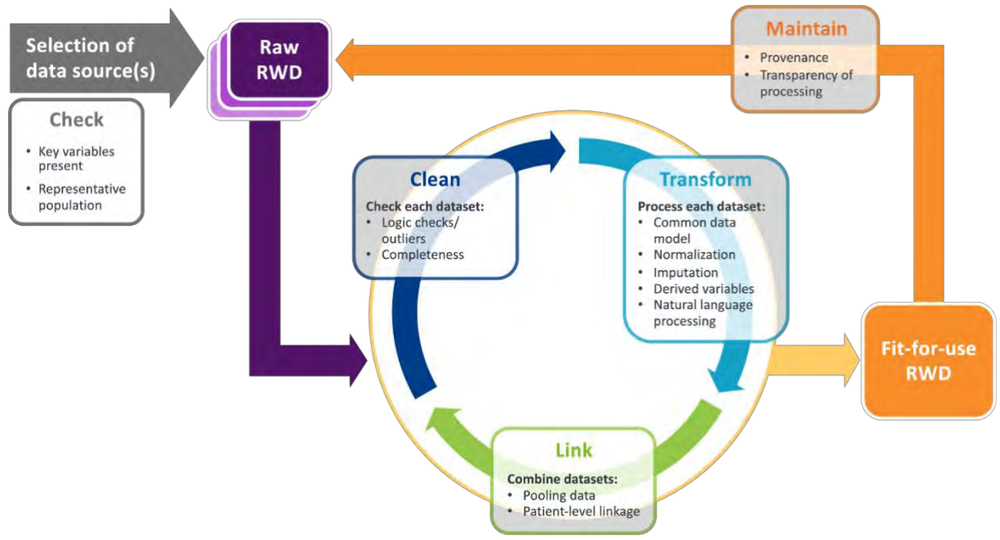
Establishing fit-for-use RWD has been a challenge because data comes from multiple sources with different formats and structures. For example, EHR data is structured differently than claims data. Additionally, data may not share the same format across various EHRs. As Figure 2 demonstrates, without standardization models, data must go through a rigorous process where it’s “cleaned,” “transformed” and “linked” before it can be deemed fit-for-use.
Without standardization models, data must go through a rigorous process where it’s “cleaned,” “transformed” and “linked” before it can be deemed fit-for-use.
Startups and Industry Initiatives Working to Push RWD/RWE Into Clinical Research and Care
Below is a list of health startups working on alleviating the data collection/analysis barriers that prevent wider implementation of RWD/RWE in clinical research and care:
RCT Duplicate: RCT Duplicate is a collaboration between Brigham and Women’s Hospital, the FDA and Aetion to replicate randomized clinical trials based on longitudinal insurance claims.
Aetion: Aetion has developed a data platform that aggregates and analyzes real world data to enable payers to determine effective treatment plans for subgroups of their member population.
Evidation: Evidation is building a data analysis platform that utilizes real time behavior data to enable new clinical insight.
Concerto HealthAI: Concerto HealthAI is using real world data and evidence to provide insight to both providers and trial Sponsors to enable precision oncology.
Nference: Nference is building a platform to enable once siloed and unstructured health data into structured data that allows for analysis and insight. Nference has a partnership with Mayo Clinic to enable more effective clinical decisions support.
OM1: OM1’s data allows stakeholders to access massive amounts of data to enable providers to more precisely treat patients based on real world evidence.
Litmus Health: Litmus Health allows for real world data collected from wearables in clinical trials to be standardized in ways that make it easy for analysis and regulatory submission
Verana Health: Verana Health is working to create the largest standardized clinical database of real world data in order to run analysis to draw insight for providers.
Flatiron Health (acquired by Roche): Flatiron Health is working to transform oncology by using real world data generated from EHRs across the US that allows researchers to use synthetic control arms in their clinical trials. Additionally, they have created an Epic App that allows for physicians to see evidence based therapy options and pulls appropriate clinical trials for each patient based on the EHR data.
Embleema: Embleema has created a platform that allows for real world data collection, analysis and data preparation for regulatory submission.
TriNetX: TriNetX allows for researchers to use its real world longitudinal data platform to accelerate clinical research..
Syapse: Syapse is transforming cancer care by utilizing real world evidence that allows physicians to make personalized care decisions.
Verantos: Verantos has created a “turn key” data platform that allows researchers to use real world data for label expansion and for care teams to run pragmatic clinical trials to develop more personalized care plans for complex patients.
COTA: COTA analyzes real world data from multiple sources to allow for providers to offer more personalized care to cancer patients.
Recommendation: Buy
In 2016, 21st Century Cures Act forced the FDA to develop guidelines on the use of RWD in medical intervention submission. In late 2018, the FDA established the Framework for the Real World Evidence Program. Additionally, in May 2019, FDA released a draft guidance for submitting RWD for the medical intervention approval process. With the combination of FDA progress, the CMS push for interoperability, and the tech advancements outlined above, use of RWD and RWE is set to rapidly mature over the next couple of years.
Resources to Learn More
FDA Framework for RWE Program
NIH Textbook on Pragmatic Clinical Trials
Friends of Cancer Research “Establishing a Framework to Evaluate Real-World Endpoints”
Using Real World Evidence to Accelerate Safe and Effective Cures
Duke “Improving the Development and Use of Real-World Data and Evidence”
Want to read more of Jumpstart’s research on clinical trials?
“Virtual Clinical Trials: Selecting Biometric Monitoring Technologies” takes a look at how technology can help reduce clinical trial costs while making them more accessible and ‘patient-centric.’
Works Cited
FDA. Real World Evidence. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence
Clinical Trials Transformation Initiative. 2019. https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/rwd-recommendations_final.pdf
Weng, C., Batres, C., Borda, T., Weiskopf, N. G., Wilcox, A. B., Bigger, J. T., & Davidson, K. W. (2011). A real-time screening alert improves patient recruitment efficiency. AMIA … Annual Symposium proceedings. AMIA Symposium, 2011, 1489–1498.
Framework For FDA’S Real-World Evidence Program. https://www.fda.gov/media/120060/download
Marra, C., Chen, J.L., Coravos, A. et al. Quantifying the use of connected digital products in clinical research. npj Digit. Med. 3, 50 (2020). https://doi.org/10.1038/s41746-020-0259-x
Baumfeld Andre, E., Reynolds, R., Caubel, P., Azoulay, L., & Dreyer, N. A. (2019). Trial designs using real‐world data: The changing landscape of the regulatory approval process. Pharmacoepidemiology and Drug Safety.
Huron Consulting Group. https://www.huronconsultinggroup.com/resources/life-sciences/value-based-contracting-in-us
UPMC Health Plan https://www.prnewswire.com/news-releases/upmc-health-plan-announces-innovative-value-based-agreement-with-astrazeneca-300785116.html
Breaking Down Barriers Between Clinical Trials and Clinical Care: Incorporating Real World Evidence into Regulatory Decision Making https://www.fda.gov/news-events/speeches-fda-officials/breaking-down-barriers-between-clinical-trials-and-clinical-care-incorporating-real-world-evidence
FDA. 2018 Drug Trials Snapshots Summary Report. https://www.fda.gov/media/120253/download
Flatiron Health. https://flatiron.com/press/press-release/flatiron-assist-epics-app-orchard/
Katkade, V. B., Sanders, K. N., & Zou, K. H. (2018). Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. Journal of multidisciplinary healthcare, 11, 295–304. https://doi.org/10.2147/JMDH.S160029
Cricket Health Whitepaper. https://www.crickethealth.com/download-white-paper/
Determining Real-World Data’s Fitness for Use and the Role of Reliability. Duke Margolis Center for Health Policy. https://healthpolicy.duke.edu/sites/default/files/u31/_determining_real-world_datas_fitness_for_use_and_the_role_of_reliability.pdf